Discovery of Electrons and Cathode Rays: Electron was discovered by J. J. Thomson in 1897
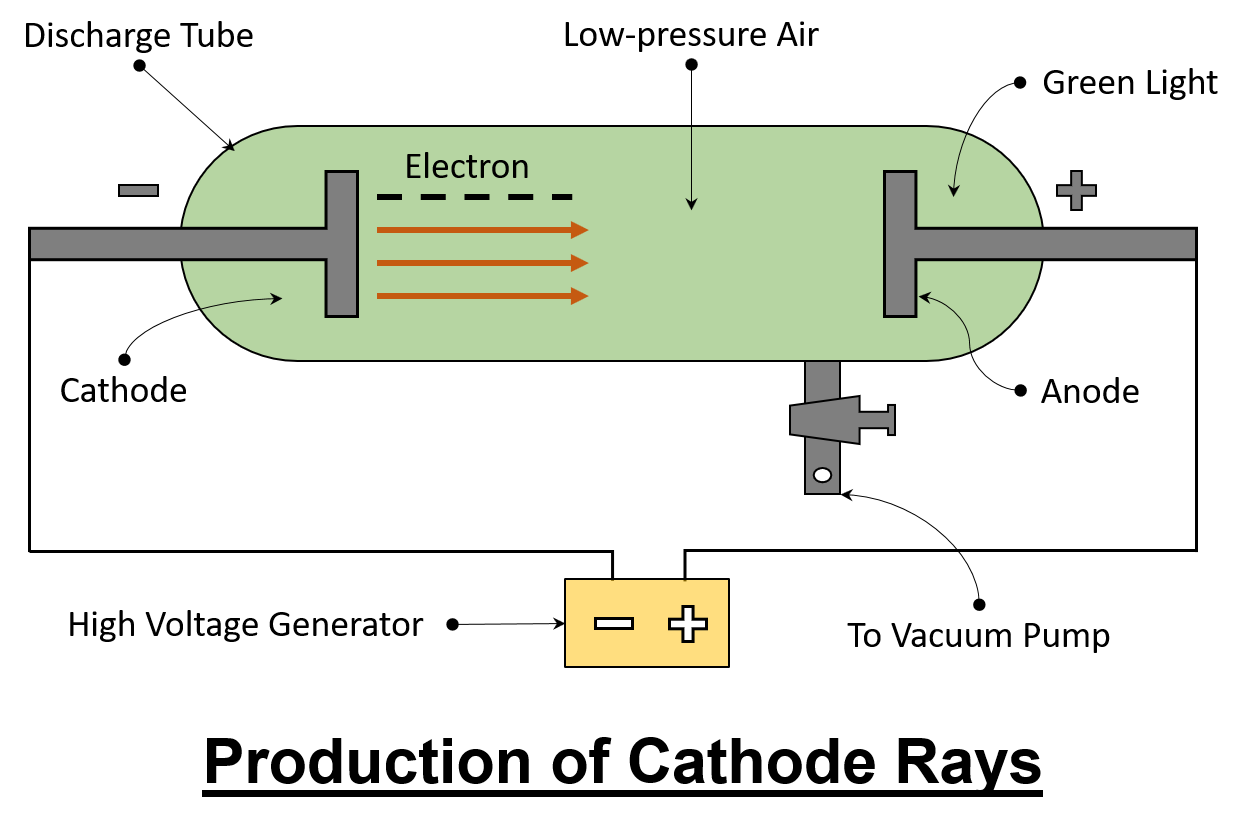
DISCHARGE TUBE Experiment: Discharge tube experiment is done by William Crookes. Discharge tube is also called "CROOCK TUBE".
Properties of Cathode Rays (Electrons): They travel in straight line from cathode towards Anode.
Introduction
- The word atom is derived from a Greek word ATOMOS means indivisible,
- which was first describe by Greek philosopher Democritus.
- Democritus belief that all matter consists of very small indivisible particles which are known as atoms.
- Johan Dalton an English school teacher and chemist suggested the fundamental atomic theory,
- which explain that all element is made up of indivisible particles called atoms.
- Dalton assumed that no particles smaller than atom exit,
- but by the passage of time new experiments show that atom is composed of even smaller particles which are known as sub-atomic particles.
- After that these sub-atomic particles were discovered and named as electron, proton and neutrons.
Discovery of Sub Atomic (Electron, Proton, Neutron) Particles of an Atom
- Dalton’s atomic theory explains the chemical nature of matter and existence of indivisible atoms.
- But at the end of 19th century sub-atomic particles were discovered by different scientists.
- First sub-atomic particle Electron discovered by M. Faraday, William Crooks and J.J. Thomson.
- Second sub-atomic particle Proton identified by Goldstein and Ernest Rutherford.
- Third sub-atomic particle Neutron revealed by Chadwick.
- All of these findings were milestone in the knowledge of atomic structure which we have now.
Discovery of Electrons and Cathode Rays
Introduction:
Electron was discovered by J. J. Thomson in 1897
Electron is the lightest particle carrying negative charge in an atom discovered by J.J. Thomson and William crooks.
Experiment: DISCHARGE TUBE
Discharge tube experiment is done by William Crookes. Discharge tube is also called "CROOCK TUBE".
In which cathode rays→ stream of electron was discovered.
Which consists of glass tube fitted with two metal electrodes connected to a high voltage source and a vacuum pump.
When electrodes inside evacuated, discharge tube are connected with high voltage source at very low pressure (10-4 atm)
As the high voltage current start passing between electrodes a streak of bluish light originates and travel in straight line from cathode (-ve electrode) to anode (+ve electrode), Which cause glow at the wall of opposite end.
These rays are called cathode rays.

After that:
- J.J. Thomson justified that these rays were deflected towards positive plate in electric and magnetic field
- which shows that these rays possess negative charge
- due to this negative charge, particle was named Electron.
- These electrons were obtained from the gas in discharge tube
- which proves that electrons are constituent of all matter.
Properties of Cathode Rays (Electrons)
1. They travel in straight line from cathode towards Anode.
2. They produce sharp shadow of an opaque object placed in their path.
3. They have negative charge and bend towards positive plate in electric and magnetic field.
4. The rays, upon striking glass or certain other materials, cause them to glow.
5. They can produce mechanical pressure indicating they possess kinetic energy (K.E).






0 Comments